Table of Contents
Introduction:
Underrated yet essential to the world of chemistry, acid-base catalysis is a pivotal procedure where an acid or a base hastens a chemical reaction while remaining unaltered. This technique is indispensable in multiple sectors, transforming unprocessed materials into valuable commodities, and plays a critical role in biological occurrences. Imagine it as the hidden component in your grandmother’s cooking that enhances all flavours – in this scenario, it facilitates quicker reactions!
Mechanism of Acid-Base Catalysis:
The machinery behind acid- and base-facilitated reactions involves a preliminary exchange of protons between an acidic catalyst and the reactant or the reactant and a basic catalyst. This phenomenon is delineated by the Bronsted-Lowry theory of acids and bases. According to the Lewis concept of acids and bases, the reaction comprises an electron pair transfer, either donated by a base catalyst or received by an acid catalyst. Picture this as a chemical greeting, where protons or electrons act as the secret codes for the handshake!
Types of Acid-Base Catalysis:
Acid-base catalysis bifurcates into two categories: general catalysis and specific catalysis. In general catalysis, all entities capable of delivering protons contribute towards reaction rate enhancement. However, in particular catalysis, the protonated solvent plays the catalyst’s role, and the reaction speed is directly linked to the number of protonated solvent molecules. Imagine the contrast between a community potluck where everyone brings a dish (general catalysis) and a dinner gathering where only the host prepares the meal (specific catalysis).
Acid-Base Catalysis in Industrial Applications:
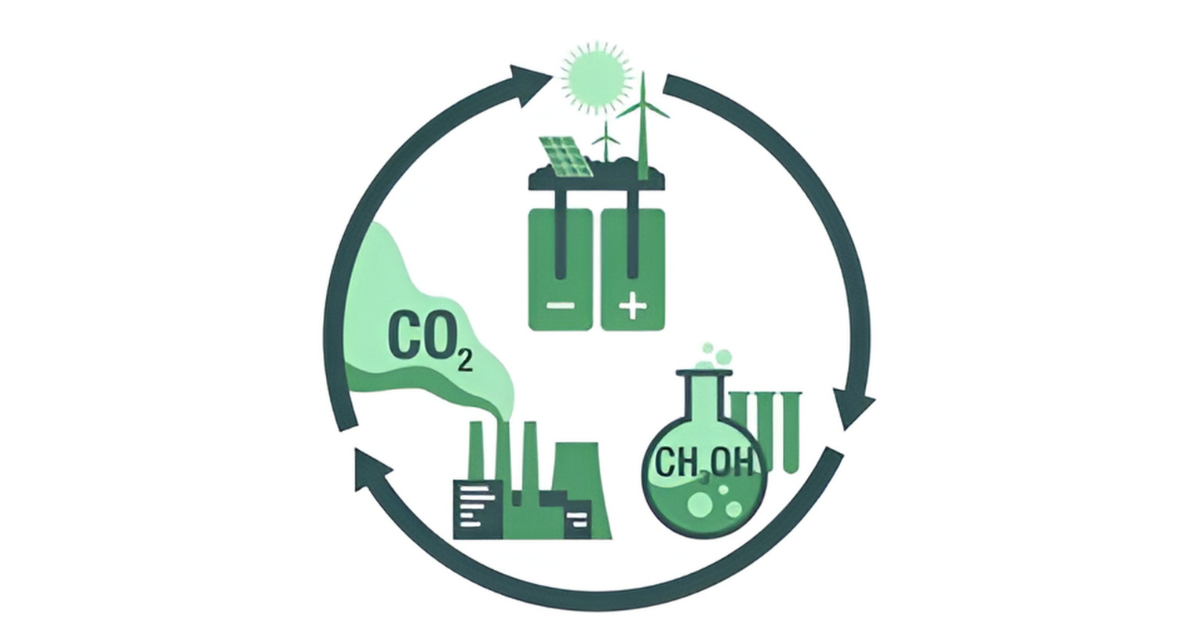
Acid catalysis finds application in numerous industrial processes, like the conversion of petroleum hydrocarbons into gasoline and various products. Heavy hydrocarbons are broken down (cracked) employing alumina-silica catalysts (Bronsted-Lowry acids), unsaturated hydrocarbons are polymerized using sulfuric acid or hydrogen fluoride (Bronsted-Lowry acids), and aliphatic hydrocarbons are rearranged (isomerized) using aluminium chloride (a Lewis acid). “Recent advancements in acid-base catalysis have led to the development of more efficient catalysts, such as those used in the electrified hydrocarbon-to-oxygenates process. This progress is a significant step towards efficient greenhouse gas mitigation. A prime example of these advancements in action is the catalytic transformation of carbon dioxide into methanol, a promising technique that not only utilizes but also reduces CO2 emissions.”
Acid-Base Catalysis in Biological Phenomena:
Within the human body, proteins, known as enzymes, serve as catalysts for several processes using general-acid and general-base catalysis. For instance, the enzyme chymotrypsin utilizes a serine hydroxyl group to initiate a nucleophilic attack on a peptide bond’s carbonyl group. This procedure, facilitated by nearby aspartic acid and histidine residues, converts serine into an alkoxide via a technique termed the charge relay system. Envision this as a relay race where the baton (proton) gets passed from one runner (amino acid) to another, eventually reaching the end goal (the reaction product).
Acid-Base Catalysis in Environmental Chemistry:
Humic acids, bountiful in natural water bodies and soil environments, can catalyze the oxidation or reduction of ions and molecules. This process is modulated by the mineral composition, solution chemistry, and microbial activity. Comprehension of this process can contribute to pollution control and mitigation. A recent application includes the Ni upcycling from electroplating wastewater to photothermal CO2 catalysis, a significant leap towards sustainable environmental management.
Conclusion:
Acid-base catalysis extends far beyond industrial applications. It bears a substantial influence on biological phenomena and environmental chemistry. By assimilating and applying this knowledge, we can augment industrial processes, develop novel pharmaceuticals or therapies, and more efficiently manage ecological pollution. Recent advancements in drug creation have witnessed the use of acid-base catalysis to create new potent and selective HSD17B13 inhibitors, which might impact disease treatment. Scientists persist in exploring novel catalysts that could facilitate the production of more energy-efficient products and decrease toxic chemical waste, thereby emphasizing the relevance and potential of acid-base catalysis in our everyday lives. Always remember, much like an excellent joke, the finest catalysts are the ones that always need to be more modern!
To conclude on a lighter note, here’s a chemistry joke: Why are chemists fond of high pH values? Because they’re in their basic element!
Check Our Chemistry Blog Here: Unravel the Secrets of Chemistry with Engaging Articles
Click Here to Dive Back into Our Educational Resources and Expand Your Knowledge Blog