Table of Contents
Acetamide: A Cosmic Connection Between Chemistry, Structure, and Practical Uses
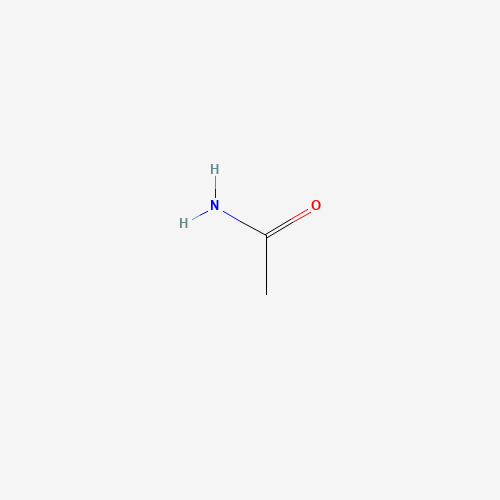
Introduction: Ah, acetamide – the life of the chemical party! While this organic compound might sound like a fancy dance move, it’s actually a derivative of acetic acid. Acetamide, also known as acetic acid amide, ethanamide, or acetamide acid, is the simplest and slightly acidic. It is widely used as an industrial solvent, plasticizer, and even in explosives (talk about living on the edge!). But did you know that it occurs naturally in red beetroot and has been detected near the center of the Milky Way galaxy? That’s right; acetamide might be the secret sauce to life in space!
Formula and Structure:
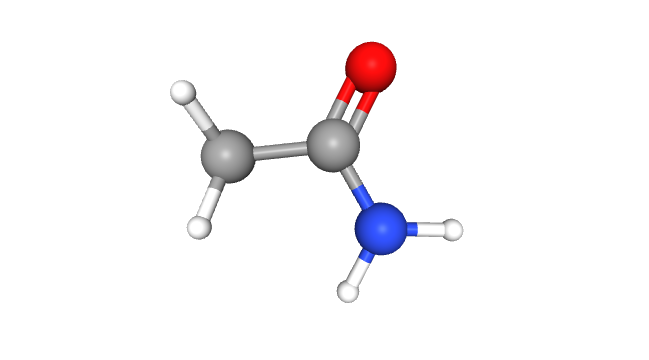
A Galactic Composition The chemical formula of acetamide is C2H5NO or CH3CONH2, and its molar mass is 59.07 g/mol – the perfect molecular weight for a chemistry superstar. The molecule comprises a methyl group (-CH3) bound to a carbonyl (CO) and an amine (NH2) group, like a cosmic conga line. The primary component of acetamide is the carboxylic acid amide functional group with the general structure RC(=O)NH2. Acetamide belongs to the family of primary carboxylic acid amides and is found naturally in specific environments.
Occurrence:
From Earth to the Milky Way, Acetamide can, be found in burning waste coal piles, forming between 50 and 150°C (122-302°F) and appearing only during periods of dry weather. It’s like acetamide is playing a game of hide-and-seek! But what’s more fascinating is that acetamide has also been detected near the center of the Milky Way galaxy. This suggests that the universe is a giant chemistry lab that cooks up amino acids in proteins and forms organic molecules in space.
Production:
The Acetamide Factory Acetamide can be produced through the ammonolysis of acetylacetone under conditions commonly used in reductive amination – a chemistry party trick! It can also be generated by dehydrating ammonium acetate or via the hydration of acetonitrile, a by-product of acrylonitrile production.
CH3CN + H2O → CH3C(O)NH2 (Party in a test tube!)
Properties:
Acetamide’s Dazzling Features Acetamide is a hygroscopic, colorless solid with a mousy odor that depends on its purity. This little party crasher has a bitter taste and is soluble in water, chloroform, hot benzene, glycerol, ethanol, and pyridine while slightly soluble in ether. It has a density of 1.159 g/cm3 and is transparent to translucent, with a white streak. Its hardness is between 1 and 1.5, similar to talc or a slightly harder substance. The melting point of acetamide ranges from 79 to 81°C, and its boiling point is 221.2°C – talk about being hot stuff!
Uses:
Acetamide’s Many Talents Acetamide is a true Renaissance compound, with numerous applications in various industries. Its unique properties make it an essential substance in chemistry and biology. Here are some of its starring roles:
- The smooth operator: Solvent for various inorganic and organic compounds
- The thrill-seeker: Component in explosives
- The influencer: Plasticizer
- The moisture magnet: Hygroscopic agent
- The creator: Manufacturing of methylamine
- The insider: Penetrating agent
- The Guardian: Stabilizer
- The firefighter: Fire suppressant
Health and Safety Hazards:
Handle with Care Although acetamide has low toxicity, it can still be a bit of a troublemaker. Exposure to high oral doses can cause weight reduction (diet, anyone?). It mildly irritates mucous membranes, eyes, and skin, and its combustion generates toxic gases or fumes. Acetamide can cause corneal damage, and repeated oral exposure over time can lead to liver tumors and lymphoma. So, remember, acetamide is fun to learn about, but it’s not a toy – handle it with care!
Conclusion:
Acetamide, the Chemistry Superstar Acetamide, is a versatile organic compound with numerous applications, making it an essential substance in chemistry and biology. Its unique properties and presence in space make it an exciting topic to explore. However, it’s crucial to handle acetamide carefully due to its potential health hazards. So, next time you come across acetamide in your chemistry adventures, remember to appreciate its fascinating characteristics and treat it with the respect it deserves!
Check Our Chemistry Blog Here: Unravel the Secrets of Chemistry with Engaging Articles
Click Here to Dive Back into Our Educational Resources and Expand Your Knowledge Blog